Introduction: Histologic transformation (HT) from follicular lymphoma (FL) to an aggressive lymphoma is infrequent but has unfavorable outcomes. Sarkozy et al. (J Clin Oncol, 2019) reported that approximately half of FL-related deaths within the first decade of diagnosis occurred following HT. Our recent analysis showed heterogeneous outcomes with median overall survival (OS) of 61 months (Day et al., ASH, 2022). Prognostic tools post HT would be clinically useful but development has been limited by small cohort sizes. Factors previously associated with outcomes following HT have included age, time to progression, prior therapy, anthracycline exposure, and early HT. Utilizing the Lymphoma Epidemiology of Outcomes (LEO) Consortium of Real World Evidence (CReWE), we describe patterns of care and evaluate other prognostic factors following HT.
Methods: This multicenter observational cohort consists of prospectively enrolled patients in the LEO Cohort, and patients with HT retrospectively identified at LEO centers. Patients diagnosed with biopsy proven grade 1-3A FL at diagnosis, and a subsequent biopsy showing HT to an aggressive lymphoma (FL grade 3B, diffuse large B cell lymphoma [DLBCL], or high-grade B cell lymphoma [HGBCL]) between 2002-2022, were eligible for the study. Patients with a component of FL grade 3B, DLBCL, or HGBCL at diagnosis were excluded. HGBCL was defined as double or triple hit or another aggressive subtype. OS was defined as the time from HT until death from any cause and calculated via Kaplan-Meier. Associations between clinical factors at time of HT and/or diagnosis and OS were evaluated using Cox models. Cause of death was evaluated using a competing risk approach.
Results: Overall, 328 patients were confirmed with HT during the study period, 41% were female, and the median age at HT was 63 years (interquartile range [IQR] 56-70). The median time from diagnosis to transformation (TDT) was 35 months (IQR 12-66). At HT, 88% had advanced stage disease (III-IV) and LDH was elevated in 49%, while few patients exhibited B symptoms (5%) and ECOG performance status was >2 in 9%.
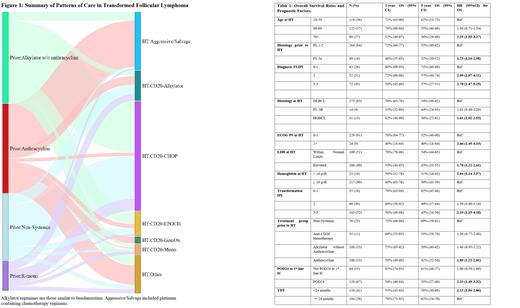
23% did not receive systemic therapy prior to HT. For those with prior therapy, the median time from prior therapy to HT was 15 months (IQR 7-39). The most frequent treatment regimens following HT were CD20-CHOP (42%), followed by platinum-based salvage therapy (20%), R-EPOCH (10%), CD20-Benda (8%), R-Mono (4%), and R-Gem-Ox (3%). 50 (15%) patients underwent consolidation with autologous stem cell transplant, 3 underwent allogeneic transplant (30 platinum-based, 15 CD20-CHOP, 5 R-EPOCH, 3 Other), and 2 received CAR T-cell therapy (Figure 1).
After median follow-up of 85 months from HT, 170 patients had died; 5-year OS was 52% and median OS was 65 months (95% CI: 55-107). Among 160 patient deaths, cause was most often lymphoma-related (progression, n=107 [63%]; therapy-related, n=14 [7%]) compared to non-lymphoma (n=20, 13%) or missing/unknown causes (n=29, 17%).
Clinical factors at the time of HT that were associated with worse OS include: ECOG PS >2, age ≥70, IPI 3-5, and shorter TDT (<24 months), hemoglobin <10 g/dl and elevated LDH. A transformation to HGBCL was also associated with inferior OS (Table 1). OS did not significantly differ by gender, BMI, stage, presence of B-symptoms, absolute lymphocyte count, number of extranodal groups, presence of bulky disease at HT, or response to prior FL treatment.
Factors at the time of FL diagnosis that were associated with decreased OS after HT included FLIPI score 3-5 (high risk), FLIPI 2 (moderate risk), and FL grade 3A at diagnosis. Exposure to any systemic therapy for FL prior to HT was associated with shorter OS, with the worst outcomes in patients receiving an anthracycline prior to HT. POD24 to frontline immunochemotherapy was associated with inferior OS after HT (Table 1).
Conclusions: Patterns of care in Transformed FL are heterogeneous and vary greatly in intensity. These data confirm previous reports that age, early HT, and any systemic therapies prior to HT are negative prognostic indicators for OS. At HT elevated LDH, low hemoglobin, POD24 status, poor performance status, and HT to HGBCL were associated with poor outcomes following HT. These data provide a basis for future prognostic indices to identify patients with highest risk of lymphoma related mortality for whom study of novel treatments is greatly needed.
Disclosures
Casulo:SecuraBio: Research Funding; GenMab: Research Funding; Abbvie: Consultancy; Follicular Lymphoma Foundation: Other: Leadership role; Bristol Myers Squibb: Consultancy, Research Funding; Gilead Sciences: Research Funding; Verastem: Research Funding; Genentech: Consultancy, Research Funding; Lymphoma Research Foundation: Other: Leadership Role. Wang:LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Morphosys: Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Nastoupil:Gilead Sciences/Kite Pharma: Honoraria, Research Funding; AstraZeneca: Honoraria; Regeneron: Honoraria; Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; AbbVie: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; DeNovo: Honoraria; Caribou Biosciences: Honoraria, Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; ADC Therapeutics: Honoraria. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Lossos:LRF: Membership on an entity's Board of Directors or advisory committees; NCI: Research Funding; Adaptive: Honoraria; NCI: Research Funding; University of Miami: Current Employment; BeiGene: Consultancy. Romancik:KITE: Consultancy; Astra Zeneca: Consultancy. Habermann:BMS: Research Funding; sorrento: Research Funding; Genentech: Research Funding. Cerhan:Genmab: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Protagonist: Other: Safety Monitoring Committee; NanoString: Research Funding; Genentech: Research Funding. Flowers:Beigene: Consultancy; 4D: Research Funding; Morphosys: Research Funding; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Genentech Roche: Consultancy, Research Funding; SeaGen: Consultancy; Acerta: Research Funding; Jannsen Pharmaceuticals: Research Funding; Bayer: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Genmab: Consultancy; Gilead: Consultancy, Research Funding; Pfizer: Research Funding; Nektar: Research Funding; Pharmacyclics: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; Spectrum: Consultancy; Pharmacyclics Jansen: Consultancy; Guardant: Research Funding; Iovance: Research Funding; Kite: Research Funding; Karyopharm: Consultancy; Celgene: Consultancy, Research Funding; Novartis: Research Funding; Ziopharm: Research Funding; Burroghs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; V Foundation: Research Funding; Adaptimmune: Research Funding; Allogene: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; CPRIT Scholar in Cancer Research: Research Funding; Denovo Biopharma: Consultancy; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Cancer Prevention and Research Institute of Texas: Research Funding; Amgen: Research Funding; Cellectis: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal